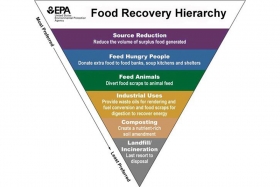
Reducing Food Waste in Foodservice
October 16, 2018 by Doreen Garelick, Dietetic Intern
Our intern Doreen attended a food waste summit for restaurants and compiled these tips to help food service operators redirect…
News Commentary
October 25, 2016

In her latest blog, Allison covers how the FDA has recently taken on the challenge to define the term "healthy." You may be surprised what sparked the action!
If you’ve been following the FDA’s food labeling news then you might feel like you have a case of déjà vu. And you actually wouldn’t be so wrong. That’s because just a few months ago, the FDA finally decided that it needed to define what it meant for a food to be “natural.” For so long, the FDA avoided pinpointing what was and wasn’t considered “natural,” but after seeing how misleading that word has been for consumers, it was finally time for them to step in. Now, the FDA has concluded that it needs to redefine what it considers “healthy.” How and why it got to this point is an interesting sequence of events, so grab yourself a “healthy” snack and read on.
It all started in March 2015 when the FDA sent a warning letter to KIND telling them that they had multiple labeling errors on their bars, including, but not limited to, the use of the word “healthy.” The FDA explained that to use the term “healthy” a food item must meet certain nutritional criteria – in this case, that it should not contain more than 1 gram of saturated fat per 40 grams of food, and it should also contain at least 10% of the Recommended Daily Intake for vitamin C, vitamin A, calcium, iron, protein, or fiber. At the time, several KIND bars did not meet this standard.
It was true; some bars had up to 5 grams of saturated fat per 40 gram serving. While technically speaking, the KIND bars did not comply with the FDA’s existing definition of “healthy,” Daniel Lubetsky, KIND’s CEO was not willing to give in so easily. Sure, he could have simply erased the term “healthy” from his labels and moved on, but that would have been like putting a Band-Aid on a deep wound. For him, this was a sign of a much larger problem. The warning letter that the FDA sent was a sign to revisit an outdated regulation.
The reason why KIND bars have such high amounts of saturated fat per Reference Amount Currently Consumed (RACC) – the technical phrase for how much Americans typically eat of a certain food – is that their main ingredient is nuts. Nuts are fats – that is, they belong to the fat food group, and while for the most part, they have unsaturated fat, some of the fat in nuts are saturated. This does not mean that nuts are an unhealthy food. In fact, most dietitians will tell you that in moderation, nuts can have a place in a healthy diet. Lubetsky likened nuts to salmon and avocado, which when eaten alone also wouldn't comply with the FDA’s standard for “healthy.” However, like nuts, salmon and avocado are inherently healthy foods with numerous nutritional benefits.
This comparison shows exactly what was flawed with the way the FDA had previously defined the use of “healthy.” By focusing on only a handful of nutrients, they excluded foods that are actually healthy, such as nuts, salmon, and avocado while letting foods that are less healthy slip by because they happen to be lower in fat, such as cereals and low fat yogurts that contain significant amounts of sugar.
So on December 1st 2015, Lubetsky launched a petition wherein he called upon the FDA to reconsider what they deem “healthy.” In his petition he states:
"Under FDA’s current application of food labeling regulations, whether or not a food can be labeled “healthy” is based on specific nutrient levels in the food rather than its overall nutrition quality. FDA formulated those regulations more than 20 years ago, when available science and federal dietary recommendations focused on limiting total fat intake. Today, these regulations still require that the majority of foods featuring a “healthy” nutrient content claim meet “low fat” and “low saturated fat” standards regardless of their nutrient density. This is despite the fact that current science no longer supports those standards."
That petition garnered so much support that in April 2016 the FDA responded to KIND with the following:
"We do not object to the specific statement that you would like to place on your bar wrappers, on the condition that there will be no other nutrition-related statement, such as express or implied nutrient content claim, on the same panel of the label…We agree with you that our regulations concerning nutrient content claims are due for a reevaluation in light of evolving nutrition research."
The last line is of utmost importance because that brings us to where we stand today. In fact, on September 27, 2016 the Director of the Office of Nutrition and Food Labeling at the FDA’s Center for Food Safety and Applied Nutrition, Douglas Balentine, Ph.D., explained the reason for this overhaul in a blog:
"As our understanding about nutrition has evolved, we need to make sure the definition for the “healthy” labeling claim stays up to date. For instance, the most recent public health recommendations now focus on type of fat, rather than amount of fat. They focus on added sugars, which consumers will see on the new Nutrition Facts label. And they focus on nutrients that consumers aren’t getting enough of, like vitamin D and potassium. By updating the definition, we hope more companies will use the “healthy” claim as the basis for new product innovation and reformulation, providing consumers with a greater variety of “healthy” choices in the marketplace."
Just as it did when it was redefining “natural” or trying to draft the new dietary guidelines, the FDA has opened up a forum for public commentary on this matter to understand what people think “healthy” actually means. The goal is to align the definition with sound public health recommendations and avoid any potential for companies to mislead consumers with unfounded labeling.
If nothing else, this story shows that change is possible. So often we feel paralyzed by rules that are antiquated or flawed. But if you truly believe in something, stand up for it and make your point heard because there could be thousands of others who share that same opinion and are looking for someone to lead the way. The FDA is looking for your thoughts to help inform their approach and instructions to submit a comment are here. Don't miss this opportunity to contribute!

October 16, 2018 by Doreen Garelick, Dietetic Intern
Our intern Doreen attended a food waste summit for restaurants and compiled these tips to help food service operators redirect food waste from landfills.
Nutrition 101

Nutrition 101
September 26, 2018 by Doreen Garelick, Dietetic Intern
Ever notice headlines about rapid weightloss? Dietetic Intern Doreen Garelick looks deeper into a recent eye-catching headline to see if there's any truth behind it.
Connect
 Follow us on Twitter
Follow us on Twitter Friend us on Facebook
Friend us on Facebook Follow us on Pinterest
Follow us on Pinterest Follow us on Instagram
Follow us on Instagram Read our Blog
Read our Blog Watch videos on YouTube
Watch videos on YouTube Watch videos on Vimeo
Watch videos on Vimeo Connect with us on Linkedin
Connect with us on Linkedin Find us on Foursquare
Find us on Foursquare
Tweets by @SPEcertifiedBlog Search
Categories
SPE Certified Newsletter
Sign up for news on the latest SPE-certified venues, events and SPE updates.
We will never share your personal information with a third party.